Jia Wei
Waters Technology (Shanghai) Co., Ltd. Experimental Center
The molecular weight of the protein drug can be determined by macroscopic characterization at the intact protein level to initially determine whether the protein expression is correct. In the BiopharmaLynx TM software, a variety of functions for the determination and characterization of the overall molecular weight of the protein have been specifically designed, which have the following characteristics.
â– Calculate the molecular weight of the protein from the raw mass spectral data.
â– Automatically label different variants of the protein.
â– Compare the difference between the sample and the standard in an intuitive way.
â– Automatic calculation of the peak intensity ratio between various modifications of the protein.
â– The interface is friendly, intuitive and easy to operate.
The calculation of molecular mass by raw mass spectrometry data is the basic function of protein molecular weight determination. In Fig. 1, the upper left is the original mass spectrometry data of immunoglobulin IgG, and the lower right is the IgG molecular mass information obtained after software analysis. With the automatic calculation capabilities of BiopharmaLynx software, complex mass spectrometry data becomes an intuitive molecular weight form. In Fig. 1, the green background color map is the molecular mass distribution data of the standard protein, and the blue background color map is the molecular mass distribution map of the sample protein. Among the results given by BiopharmaLynx, IgG has multiple molecular mass forms due to its inclusion of multiple glycosylation modifications.
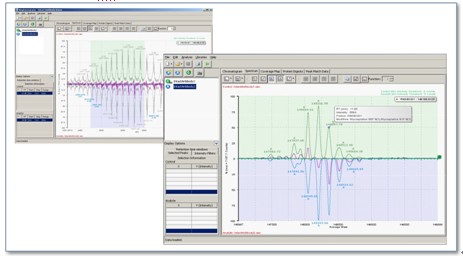
Figure 1. Complete protein amount analysis interface for BiopharmaLynx software.
The purple lines in the figure visually show the difference in the mass distribution of the sample protein and the standard. Observing the purple line morphology, it can be found that the sample IgG has more macromolecular glycosylation modified forms, while the small molecular weight glycoforms in the standard protein are more modified. When the mouse pointer is placed at the tip of the peak, information such as the protein name, modification type, peak intensity, and chromatographic retention time will automatically appear here. Through the above two kinds of information, the difference between the two can be found simply and intuitively.
BiopharmaLynx software automatically labels different modifications of the protein based on user settings. In addition to the built-in 90 modifiers, users can create their own modifications as needed. In particular, considering the specifics of bioprotein drugs, BiopharmaLynx has built-in protein modification modifications common to protein-expressing drugs, such as the loss of Lysine at the C-terminus of the protein (Fig. 2, red arrow pointing). These details will help users greatly improve their work efficiency and save energy.
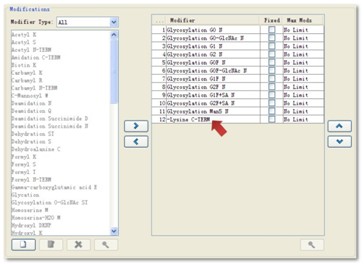
Figure 2. The grooming setup interface using BiopharmaLynx software.
BiopharmaLynx software can also visually give preliminary analysis of the ratio of various modifications of the protein (Figure 3).
As a leader in both liquid and mass spectrometry, Waters offers a comprehensive range of protein molecular weight analysis solutions, including chromatographic columns, chromatographic gradient methods, and mass spectrometry conditions. 4). Using this holistic solution, using only 0.5 micrograms of IgG protein, the entire process of liquid data collection can be completed in 4 minutes. This protocol also includes an analytical method for IgG after reduction (Figure 4, top right).
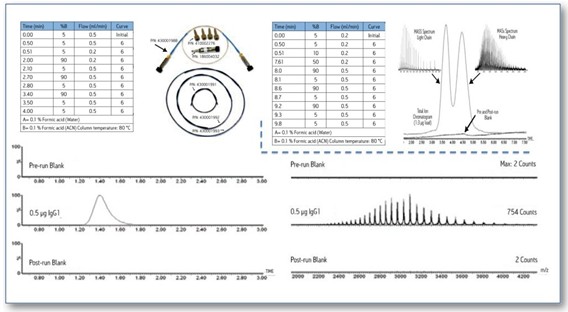
Figure 4. Schematic diagram of the complete and reduced IgG mass determination solution.
references
(1) Rapid Profiling of Monoclonal Intact Antibodies by LC/ESI-TOF MS. Waters Application Note, 2007, 720002393 EN
(2) Rapid Screening of Reduced Monoclonal Antibodies by LC/ESITOF MS. Waters Application Note, 2007, 720002394 EN
(3) Characterization of an IgG1 Monoclonal Antibody and Related Sub-Structures by LC/ESI-TOF MS, 2007, 720002107 EN
(4) Assessing the Quality and Precision of T herapeutic Antibody LC/MS Data Acquired and Processed using Automated Workflows. Poster presented at the ASMS meeting. 2008, 720002687 EN
(5) Efficiently Comparing Batc hes of an Intact Monoclonal Antibody using t he Biop harma Lynx Software Package. Waters Application Note, 2008, 720002820 EN
Piston Pin
it is full range of piston pin for the following type :
AAA) YANMAR DIESEL ENGINE
K(D)L, KFL-T, 6MAL-H/T/HT/DT, S165-T/ST/EN,
S185-ST/UT/ET, M200L-UN/SN/EN/M220L-UN/SN/EN / N 330
BBB) DAIHATSU DIESEL ENGINE
PS22/26/ DS18(A)/ DS(B)22/
DL20/22/28/ DK20 / DK 28
CCC) NIIGATA DIESEL ENGINE
6L(M)25BX/6M28AFTE/BX/6M31X/EZ/EX/ 31AFTE /34AGT
DDD) AKASAKA
AH30/AH38/ AH40/D/F/ A31/34/37/41/ DM28/30/33/36/38/40(A)K/ DM46/ UEC37H-HB/ UEC 37/88H
UEC 37LA/ UEC45LA/ UEC52LA/ UEC52HA/ UEC60HA/ UEC60LS/ UEC45HA/115 ALL UET TYPE
EEE) MITSUBISHI (KOBE AND AKASAKA )
45/75C/ 45/80D/ 52/90D/ 52/105D.E/
UEC37H-IIB/ UET37/88HA/UEC45HA.LA/ UEC52HA.LA/ S6B/
ETC
FFF) HANSHIN
6LU(D)26/ 6LU(N)28A/ 6LU(D)32/6LU(D)35/ 6LU(S)38/ 40 / 6LU40/ 6LU46A/ 6LU50A/
6EL30/ EL(S)32 /6EL(S)35/ 6EL38/ 6EL40/ 6EL(S)44/50A/
GGG) MAN B&W
S35MC/L35MCE/L50MCE/ L60MCE/ 40/54A
HHH) MITSUBISHI PURIFIER
SJ700~SJ1800/SJ 2000
III)SULZER
RND 68 RND 76 RD 44
JJJ) MAKITA
GSLH – 633 637 KSLH -633 637
Kkk) PIELSTICK
PC 2-5 PC 2-6
Piston Pin,Standard Piston Pin,Piston Pin Material,Engine Piston Pin
ZhouShan HeCheng Machinery Co., LTD. , https://www.hcmarineparts.com


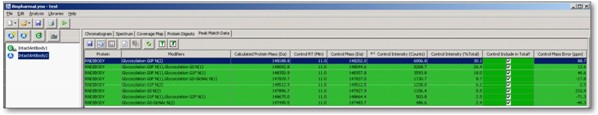
 Figure 4. Schematic diagram of the complete and reduced IgG mass determination solution.
Figure 4. Schematic diagram of the complete and reduced IgG mass determination solution.