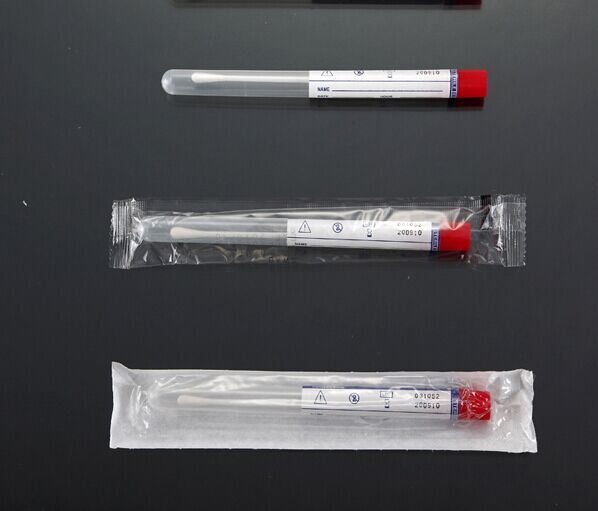
(1). Manufactured from virgin polypropylene or LDPE tubes, round bottom with size Dia. 13mm x H 150mm
(2). Ideal for sample collection and transport device for bacteria
(3). CE Marked as Class Iia in accordance with the Medical Device Directive 93/42/EEC
(4). Colour coded caps for easier identification

Â
|
Transport Swabs, Without Medium: | ||||||
| Cat. No. | Material Stick/Tip | Stick/Tip | Packaging Option | Case Qty | Sterility |
 |
| 72300 | PP, Rigid | PS/Cotton | 100/box | 1000 | NON- |
 |
| 72310 | PP, Rigid | PS/Cotton | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72330 | PP, Rigid | PS/Cotton | 1/peel, 100peels/box | 1000 | EO |
 |
| 72320 | PP, Rigid | PS/Cotton | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72400 | PP, Rigid | PS/Viscose | 100/box | 1000 | NON- |
 |
| 72410 | PP, Rigid | PS/Viscose | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72430 | PP, Rigid | PS/Viscose | 1/peel, 100peels/box | 1000 | EO |
 |
| 72420 | PP, Rigid | PS/Viscose | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72410PP | PP, Rigid | PP/Viscose | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72700 | LDPE, Flexible | PS/Cotton | 100/box | 1000 | NON- |
 |
| 72710 | LDPE, Flexible | PS/Cotton | 1/peel, 100peels/box | 1000 | EO |
 |
| 72720 | LDPE, Flexible | PS/Cotton | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72800 | LDPE, Flexible | PS/Viscose | 100/box | 1000 | NON- |
 |
| 72810 | LDPE, Flexible | PS/Viscose | 1/peel, 100peels/box | 1000 | EO |
 |
| 72820 | LDPE, Flexible | PS/Viscose | 1/flow pack, 100packs/box | 1000 | EO |
 |
(1). Manufactured from virgin polypropylene or LDPE tubes, round bottom with size Dia. 13mm x H 150mm
(2). Ideal for sample collection and transport device for bacteria
(3). CE Marked as Class Iia in accordance with the Medical Device Directive 93/42/EEC
(4). Colour coded caps for easier identification


Â
|
Transport Swabs, Without Medium: | ||||||
| Cat. No. | Material Stick/Tip | Stick/Tip | Packaging Option | Case Qty | Sterility |
 |
| 72300 | PP, Rigid | PS/Cotton | 100/box | 1000 | NON- |
 |
| 72310 | PP, Rigid | PS/Cotton | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72330 | PP, Rigid | PS/Cotton | 1/peel, 100peels/box | 1000 | EO |
 |
| 72320 | PP, Rigid | PS/Cotton | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72400 | PP, Rigid | PS/Viscose | 100/box | 1000 | NON- |
 |
| 72410 | PP, Rigid | PS/Viscose | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72430 | PP, Rigid | PS/Viscose | 1/peel, 100peels/box | 1000 | EO |
 |
| 72420 | PP, Rigid | PS/Viscose | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72410PP | PP, Rigid | PP/Viscose | 1/peel, 100peels/box | 1000 | IR | CE0197 |
| 72700 | LDPE, Flexible | PS/Cotton | 100/box | 1000 | NON- |
 |
| 72710 | LDPE, Flexible | PS/Cotton | 1/peel, 100peels/box | 1000 | EO |
 |
| 72720 | LDPE, Flexible | PS/Cotton | 1/flow pack, 100packs/box | 1000 | EO |
 |
| 72800 | LDPE, Flexible | PS/Viscose | 100/box | 1000 | NON- |
 |
| 72810 | LDPE, Flexible | PS/Viscose | 1/peel, 100peels/box | 1000 | EO |
 |
| 72820 | LDPE, Flexible | PS/Viscose | 1/flow pack, 100packs/box | 1000 | EO |
 |
Total knee replacement is for patients with severe pain and/or severe joint disability due to osteoarthritis, post-traumatic arthritis, rheumatoid arthritis, or previous implant failure.
The total knee joint system consists of femoral condylar , tibial tray,tibial insert and patella.The femoral condylar and tibia tray are made of Co-Cr-Mo in accordance with the quality standards.Tibial insert and patella are made of UHMWPE containing PBHP antioxidants.
The revision knee system is suitable for cemented total knee replacement in patients with severe joint pain or severe disability due to osteoarthritis, post-traumatic arthritis, or rheumatoid arthritis, moderate knee varus, varus, or flexion deformity of the knee, avascular necrosis of the femoral condyle, previous failed knee replacement, osteotomy, or other knee surgery.
Total knee arthroplasty (TKA) is a practical and effective method for the treatment of serious knee arthropathy. However, attention should be paid to the selection of indications, the correction of joint internal and external inversion and flexion deformity, and the correct placement of tibial prosthesis to reduce the loosening of tibial prosthesis. At the same time, early functional exercise should be carried out to reduce postoperative complications. Total knee arthroplasty (TKR) provides an effective treatment for patients with severe knee arthrosis.
knee joint replacement,total knee replacement,knee replacement revision,knee replacement procedure
Jiangsu Aomed Ortho Medical Technology Co.,Ltd , https://www.medthofixation.com